Limestone is heated and decomposed into lime. Because of the difference of kiln type, raw material, fuel type and calcination condition, the activity of lime is very different. How to increase the activity of lime in the production process, let me take you to understand the active lime below.
The activity of lime is related to the chemical composition and lithofacies composition of limestone. The harmful impurities in limestone are: SiO 2, AlO 3, FeO 3, NaO 2, KO 2, P, S, etc. These impurities react with calcined lime (CaO) at lower temperature (900 ℃), which promotes the fusion of CaO particles and leads to the coarsening of the crystallization of the particles. Iron compounds and aluminium compounds are strong flux, which can promote the formation of fusible calcium silicate, calcium aluminate and calcium ferrite. These molten compounds will block the fine pore on the surface of lime and reduce the reactivity of lime. They will also block the discharge of CO2 gas and form raw lime in some parts of the center. More importantly, they will react with lime to form slag blocks, which will make the condition of lime kiln imbalance and seriously reduce the activity of lime.
The activity of lime refers to the reaction ability of lime with other substances in slag, which is expressed by the melting rate of lime in slag. Because it is difficult to directly measure the melting rate (thermal activity) of lime in molten slag, the reaction rate of lime and water, i.e. the activity of lime-water, is usually used. The results show that the reaction rate between lime and water reflects the melting rate of lime in the slag. Therefore, the water activity of lime has been used as one of the quality indicators of lime.
There are free calcium oxide and bound calcium oxide in the lime composition, and active calcium oxide and inactive calcium oxide in the free calcium oxide. Under ordinary digestion conditions, inactive calcium oxide can not react with water, but it may be converted into active calcium oxide (e.g. after grinding). Active calcium oxide is the part of free calcium oxide which can react with water under ordinary digestion conditions. Combined with calcium oxide is irreversible, so it can not be called inactive calcium oxide. Reactivity of lime can be seen as the amount of active calcium oxide in the total amount of free calcium oxide.
Calcination of limestone is a process of recrystallization of rhombic lattice of limestone into cubic lattice of lime. The crystal structure obtained by the change is related to the growth rate of the new phase nucleus. When the former is larger than the latter, fine-grained crystals are obtained, which have a large number of active calcium oxide molecules and high surface energy. On the contrary, coarse-grained crystals with low surface energy have a small number of active calcium oxide molecules. The activity of lime with fine crystal structure is high when calcined by rapid heating, while the activity of lime with coarse crystal structure is low when calcined by slow heating.
CaO, Fe2O3, CaO, Al2O3, 2CaO, SiO 2 and CaSO4 salts are easily formed in the solid fuel during the calcination process. They are coated on the surface of lime particles and form a slag film, which slows down the decomposition rate of lime and results in the formation of inactive lime. However, if the operating conditions and raw materials and fuel conditions are improved, the activity of lime can reach about 300 ml. The ideal firing temperature range is 1100 ~ 1200 ℃, and the activity of lime is higher.
The influence of storage time and transportation mode on the activity of lime was studied. The lime from kiln was stacked into cubes and the activity of lime was sampled daily. With the prolongation of storage time, the activity of lime decreases. Lime used in steelmaking is transported by canopy truck. Lime activity also decreases during transportation. Transportation is more severe in the rain. The reason is that lime absorbs water in the air and digests itself to form Ca (OH) 2, which then reacts with CO2 in the air and becomes insoluble CaCO 3, thus reducing the activity of lime. It can be seen that the sealing device should be used in the storage and transportation of active lime.
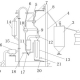
There are many factors to increase the activity of lime. A set of mature scientific and economic active lime production device can save a lot of trouble. With the continuous development of science and technology, the traditional production process needs to be constantly updated or eliminated. The activated lime Suspension Calcination developed by Luoyang Building Materials Architectural Design Research Institute is technologically advanced and can realize other traditional production processes that can not be realized. The process steps are as follows: (1) In production, the induced draft fan and the high temperature fan suck through the combined pipe to produce the running air flow, the air passes through the intake pipe, then enters the grading cooling device, then enters the roaster through the fifth pipe, the air flow enters the cyclone separator through the L-tube at the upper end of the roaster, and then passes through the third preheating ascending flue, the third cyclone preheater, and so on. The second preheating ascending flue, the second cyclone preheater, the first preheating ascending flue, the first cyclone preheater, the exhaust flue, the waste heat boiler, the dust removal device and the induced draft fan are discharged from the chimney; (2) While the airflow is running, the fuel enters the burner through the fuel inlet pipeline, and then sprays fuel into the calciner. The fuel burns in the calciner, and the airflow is heated to the calcination temperature. The hot air is discharged from the upper part of the roaster, and then passed through the cyclone separator, followed by the third preheating ascending flue, the third cyclone preheater, the second preheating ascending flue, the second cyclone preheater, the first preheating ascending flue and the first cyclone preheater. After heat transfer and cooling with the incoming lime powder, the hot air enters the exhaust flue and then enters the waste heat boiler for further cooling. (3) Limestone material enters into the pulverizing device through the tube of the limestone material enters into the pulverizing device, after which the powdery limestone material is fed into the silo, and then into the first preheating rising flue through the metering device and the feeding device, and then through the first cyclone preheater, the second preheating rising flue and the second swirl in turn. The air preheater, the third preheating ascending flue and the third cyclone preheater enter the roasting furnace after preheating with the exhausted hot air. After thorough decomposition at roasting temperature, CaCO 3 is decomposed into CaO and CO. The roasted active lime enters the cyclone separator with the hot air flow for gas-solid separation. Fourthly, the separated active lime powder flows through the sixth pipeline into the stage cooling. The device, through heat exchange and cooling with the incoming air, enters the ash outlet tube and feeds it into the finished product warehouse. After preheating at this stage, the air enters the calciner for combustion support.
Activated lime Suspension Calcination developed by Luoyang Building Material and Architectural Design and Research Institute has large single-line capacity, uniform heating, rapid reaction, high product activity, no waste, low investment and operation cost. It can greatly reduce the problems caused by technology, equipment and human factors in the production process, save costs and increase production at the same time. It provides strong technical support and help for the active lime industry.